The Top 5 Reasons Practices Are Moving to mild® After the First ESI Fails
Published July 21, 2021
Today, an increasing number of practices recommend their patients move to mild® after the first epidural steroid injection (ESI) fails, and it’s easy to see why. mild® provides patients relief from the symptoms of lumbar spinal stenosis (LSS) and has a safety profile equivalent to an ESI, but with lasting results. In the following article, we present the most common reasons physicians and Advanced Practice Providers (APPs) are moving their patients to mild® earlier in the treatment journey.
Ready to move to mild®? Skip to the end for 3 pragmatic solutions you can implement in your practice today to get started, and get your patients on the path to relief.
5-year durability
A recently published study conducted by the Cleveland Clinic Study explores how many patients were able to avoid surgical decompression after the mild® Procedure over a five-year period.
The data showed that 88% of patients avoided surgical decompression for at least 5 years.
The study authors concluded that “the durability of mild® over 5 years may allow elderly patients with symptomatic lumbar spinal stenosis to avoid lumbar decompression surgery while providing significant symptomatic relief.” They also noted that “because the mild® Procedure demonstrated durability up to 5 years, it might also be speculated with caution, that appropriate patients should be encouraged to undergo the mild® Procedure as early as needed, rather than waiting until these patients are at an advanced age.”
mild® removes the problem and leaves nothing behind
No implants. mild® removes a major root cause of neurogenic claudication by debulking the hypertrophic ligamentum flavum, which reduces the compression of the nerves without leaving any implants behind.
No stitches. The entire procedure can be performed through a single, tiny incision the size of a baby aspirin (5.1mm).

mild® does not significantly alter the structural anatomy of the spine or eliminate future treatment options
Safety profile equivalent to an ESI
mild® offers a clinically proven safety profile equivalent to an ESI, even in patients with comorbidities.,
No device- or procedure-related serious adverse events or complications have been reported in any clinical trial.
Patients typically resume normal activity within 24 hours with no restrictions
Unlike more invasive procedures that can require a lengthy recovery, mild® patients typically resume normal activity within 24 hours with no restrictions.

“When I went for the procedure, it was any other day. I went into the procedure. You feel nothing, and then you come back and you’re fine. I mean, it’s no big deal. There’s no pain, there’s no recovery, you just go home.”
-Nicky, mild® Patient

“By the time I walked out [after the mild® Procedure], I went home, and I could get out. The first thing I wanted to do was start doing stuff. That was a big moment in my life emotionally.”
-Ronnie, mild® Patient
Avoids Epidural Exhaustion
LSS patients who receive a series of epidural injections may become frustrated or start to lose hope if the injections provide little relief or decreasing relief over time. While ESIs can sometimes deliver transient, temporary relief, they do not address the root cause of stenosis. The mild® Procedure decompresses, debulking the hypertrophied ligament, to reduce spinal canal narrowing, giving patients a new option to achieve long-term relief.
Featured Data: Long-Term Effects of Repeated Injections of Local Anesthetic With or Without Corticosteroid for Lumbar Spinal Stenosis: A Randomized Trial
Janna L. Friedly et al. studied the long-term effectiveness of ESIs for LSS and the effect of repeat injections. The trial concluded that repeated epidural injections offer no additional benefit if injections in the first 6 weeks did not improve pain.

Physician perspective: Alexander Escobar, MD
“If the patient has received ESIs in your practice and the outcomes were not efficacious, there is no reason to offer them another one. If a patient comes to you from another practice, and has recent history of epidural steroid injections, move to mild®. They’re likely seeking an alternate option, and mild® is something that can provide them hope.”

Patient perspective: Lynn, mild® Patient
“The first epidural lasted about three months and then the pain was back. I went for the second epidural, and it didn’t last two weeks. My physician said, ‘Well, you can have one more.’ I said, ‘No, I’m finished with them, I want the mild® Procedure.’”
Ready to move to mild®?
Here are a few tips to help you successfully integrate mild® into your practice.
1. Look for the ligament, it’s a common problem
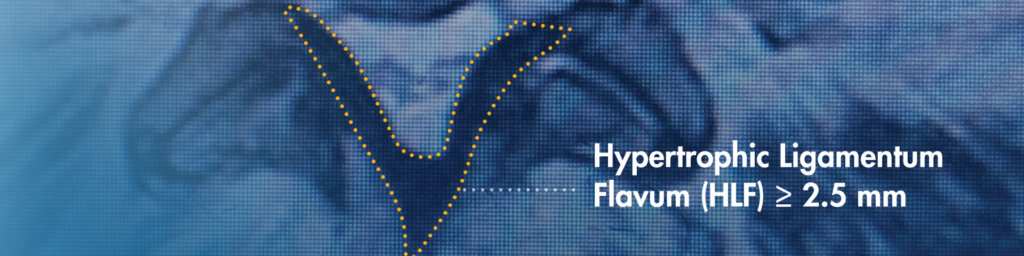
When reviewing a patient’s MRIs/CTs, be sure to identify hypertrophic ligamentum flavum (HLF). HLF ≥ 2.5 mm can be readily identified and is surprisingly common, contributing to up to 85% of spinal canal narrowing.
2. Present mild® in initial ESI treatment plan
Introducing patients to mild® early in their treatment journey can help them feel assured that they have effective options if ESIs don’t provide the relief they need. Dr. Jason Pope of the Evolve Restoration Center confirms, “By presenting mild® as part of the treatment plan from the start, patients have more confidence that we are going to be proactive in their care, and make sure they have the opportunity to access advanced LSS treatment options that offer excellent outcomes.”
3. Engage APPs in patient identification and treatment planning
Many practices that offer mild® ensure that physicians and Advanced Practice Providers (APPs) alike are trained to identify patient candidates who may benefit from the mild® Procedure. Jane Hartigan, an APP in a leading Northern California pain management practice recommends asking proactive questions like, “Does hunching forward significantly relieve the pain you’re feeling?”, or “Do you feel pain in your back and legs when standing or walking?” By understanding your patient’s current experience with LSS, their treatment history, and functional goals, you can readily spot the many patients in your practice who may benefit from the mild® Procedure.
Benyamin RM, Staats PS, MiDAS ENCORE Investigators. mild® is an effective treatment for lumbar spinal stenosis with neurogenic claudication: MiDAS ENCORE Randomized Controlled Trial. Pain Physician. 2016;19(4):229-242.
Mekhail N, Costandi S, Abraham B, Samuel SW. Functional and patient-reported outcomes in symptomatic lumbar spinal stenosis following percutaneous decompression. Pain Pract. 2012;12(6):417-425. doi:10.1111/j.1533-2500.2012.00565.x.
2012 data from Health Market Sciences report for Vertos Medical 2013.
Data on file with Vertos Medical.
Staats PS, Chafin TB, Golvac S, et al. Long-term safety and efficacy of minimally invasive lumbar decompression procedure for the treatment of lumbar spinal stenosis with neurogenic claudication: 2-year results of MiDAS ENCORE. Reg Anesth Pain Med. 2018;43:789-794. doi:10.1097/AAP.0000000000000868.
Based on mild® Procedure data collected in all clinical studies. Major complications are defined as dural tear and blood loss requiring transfusion.
MiDAS ENCORE responder data. On file with Vertos Medical.
Jain S, Deer TR, Sayed D, et al. Minimally invasive lumbar decompression: a review of indications, techniques, efficacy and safety. Pain Manag. 2020;10(5). https://doi.org/10.2217/pmt-2020-0037. Accessed June 1, 2020.
Deer TR, Grider JS, Pope JE, et al. The MIST Guidelines: the Lumbar Spinal Stenosis Consensus Group guidelines for minimally invasive spine treatment. Pain Pract. 2019;19(3)250-274. doi:10.1111/papr.12744.
Hansson T, Suzuki N, Hebelka H, Gaulitz A. The narrowing of the lumbar spinal canal during loaded MRI: the effects of the disc and ligamentum flavum. Eur Spine J. 2009;18(5):679-686. doi:10.1007/s00586-009-0919-7.
Treatment options shown are commonly offered once conservative therapies (e.g., physical therapy, pain medications, chiropractic) are not providing adequate relief. This is not intended to be a complete list of all treatments available. Doctors typically recommend treatments based on their safety profile, typically prioritizing low risk/less aggressive procedures before higher risk/more aggressive procedures, but will determine which treatments are appropriate for their patients.
The mild® Procedure is a minimally invasive treatment for lumbar spinal stenosis. As with most surgical procedures, serious adverse events, some of which can be fatal, can occur, including heart attack, cardiac arrest (heart stops beating), stroke, and embolism (blood or fat that migrates to the lungs or heart). Other risks include infection and bleeding, spinal cord and nerve injury that can, in rare instances, cause paralysis. This procedure is not for everyone. Physicians should discuss potential risks with patients. For complete information regarding indications for use, warnings, precautions, and methods of use, please reference the devices’ Instructions for Use.
Patient stories on this website reflect the results experienced by individuals who have undergone the mild® Procedure. Patients are not compensated for their testimonial. The mild® Procedure is intended to treat lumbar spinal stenosis (LSS) caused by ligamentum flavum hypertrophy. Although patients may experience relief from the procedure, individual results may vary. Individuals may have symptoms persist or evolve or other conditions that require ongoing medication or additional treatments. Please consult with your doctor to determine if this procedure is right for you.
Reimbursement, especially coding, is dynamic and changes every year. Laws and regulations involving reimbursement are also complex and change frequently. Providers are responsible for determining medical necessity and reporting the codes that accurately describe the work that is done and the products and procedures that are furnished to patients. For this reason, Vertos Medical strongly recommends that you consult with your payers, your specialty society, or the AMA CPT regarding coding, coverage and payment.
Vertos Medical cannot guarantee coding, coverage, or payment for products or procedures. View our Billing Guide.
Vertos is an equal employment opportunity workplace committed to pursuing and hiring a diverse workforce. We strive to grow our team with highly skilled people who share our culture and values. All qualified applicants will receive consideration for employment without regard to sex, age, color, race, religion, marital status, national origin, ancestry, sexual orientation, gender identity, physical & mental disability, medical condition, genetic information, veteran status, or any other basis protected by federal, state or local law.
Hall S, Bartleson JD, Onofrio BM, Baker HL Jr, Okazaki H, O’Duffy JD. Lumbar spinal stenosis. Clinical features, diagnostic procedures, and results of surgical treatment in 68 patients. Ann Intern Med. 1985;103(2):271-275. doi:10.7326/0003-4819-103-2-271.
Kalichman L, Cole R, Kim DH, et al. Spinal stenosis prevalence & association with symptoms: The Framingham Study. Spine J. 2009;9(7):545-550. doi:10.1016/j.spinee.2009.03.005.
Fukusaki M, Kobayashi I, Hara T, Sumikawa K. Symptoms of spinal stenosis do not improve after epidural steroid injection. Clin J Pain. 1998;14(2):148-151. doi:10.1097/00002508-199806000-00010.
Mekhail N, Costandi S, Nageeb G, Ekladios C, Saied O. The durability of minimally invasive lumbar decompression procedure in patients with symptomatic lumbar spinal stenosis: Long-term follow-up [published online ahead of print, 2021 May 4]. Pain Pract. 2021;10.1111/papr.13020. doi:10.1111/papr.13020
Friedly JL, Comstock BA, Turner JA, et al. Long-Term Effects of Repeated Injections of Local Anesthetic With or Without Corticosteroid for Lumbar Spinal Stenosis: A Randomized Trial. Arch Phys Med Rehabil. 2017;98(8):1499-1507.e2. doi:10.1016/j.apmr.2017.02.029
Pope J, Deer TR, Falowski SM. A retrospective, single-center, quantitative analysis of adverse events in patients undergoing spinal stenosis with neurogenic claudication using a novel percutaneous direct lumbar decompression strategy. J Pain Res. 2021;14:1909-1913. doi: 10.2147/JPR.S304997
Pryzbylkowski P, Bux A, Chandwani K, et al. Minimally invasive direct decompression for lumbar spinal stenosis: impact of multiple prior epidural steroid injections [published online ahead of print, 2021 Aug 4]. Pain Manag. 2021;10.2217/pmt-2021-0056. doi:10.2217/pmt-2021-0056
Abstract presented at: American Society of Pain and Neuroscience Annual Conference; July 22-25, 2021; Miami Beach, FL.
Mobility Matters: Low Back Pain in America, Harris Poll Survey, 2022. View data and full summary here.
Deer TR, Grider JS, Pope JE, et al. Best Practices for Minimally Invasive Lumbar Spinal Stenosis Treatment 2.0 (MIST): Consensus Guidance from the American Society of Pain and Neuroscience (ASPN). J Pain Res. 2022;15:1325-1354. Published 2022 May 5. doi:10.2147/JPR.S355285.

