PSPS Abstract: Patients with Foraminal Narrowing Benefit From mild® Treatment
Source— PSPS 2021 Abstract Author— Denis Patterson Published October 11, 2021
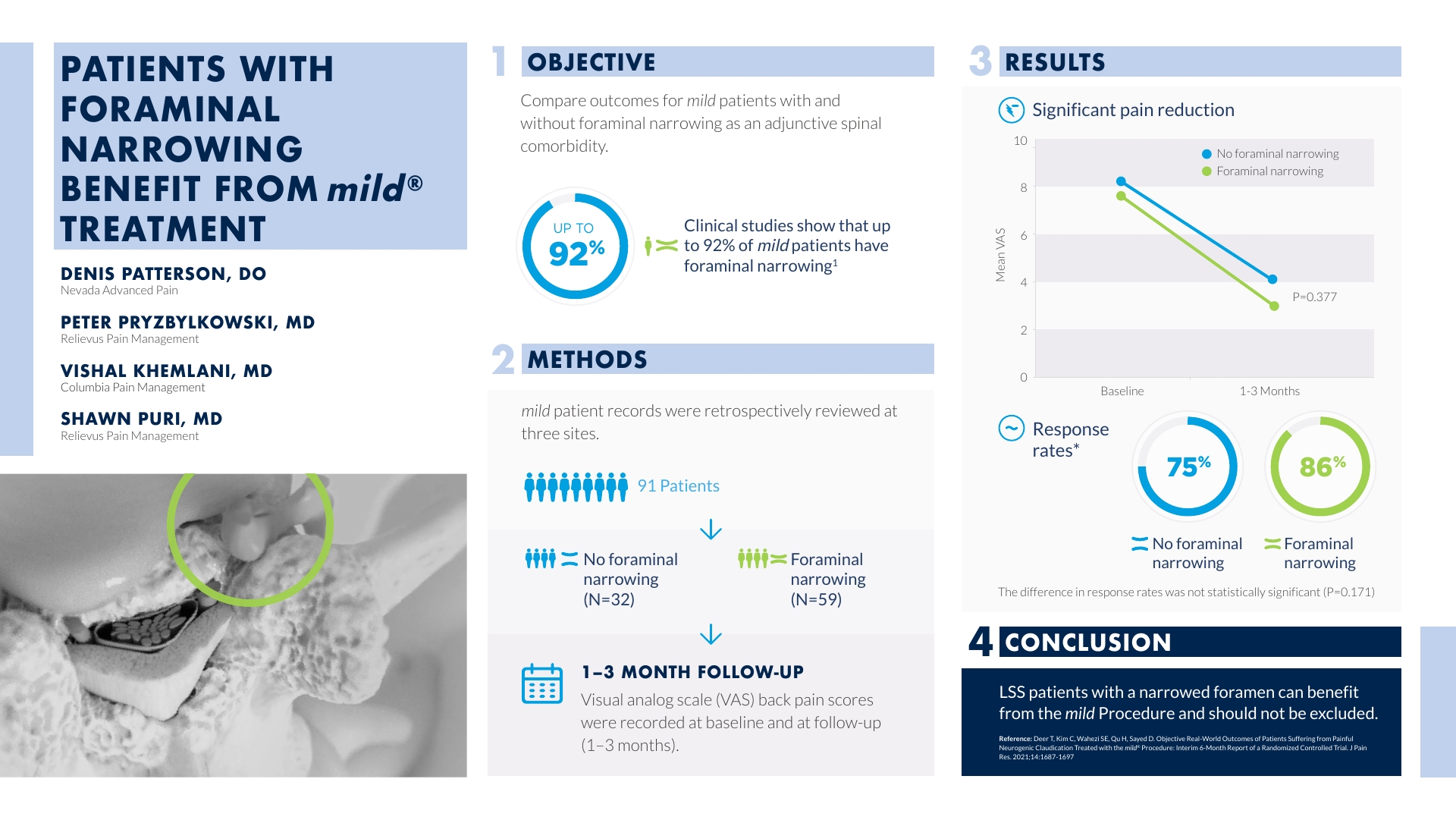
The following study, “Patients with Foraminal Narrowing Benefit from mild® Treatment” investigates procedural outcomes for mild® patients with and without foraminal narrowing as an adjunctive spinal comorbidity. Ninety-one patients from three sites were accepted for analysis and VAS scores were compared within each group over time and between groups. Responder rates were 86.4% in the group with foraminal narrowing, compared to 75.0% in the group having no foraminal narrowing. Though the amount of pain improvement between groups was not significantly different, this study confirms that lumbar spinal stenosis (LSS) patients with a narrowed foramen can benefit from the mild® Procedure and should not be excluded from treatment. View the abstract poster below to learn more about the results.
Watch Dr. Denis Patterson present his abstract from the Pacific Spine & Pain Society (PSPS) 2021 Annual Conference where he reviews the data and shares why LSS patients with a narrowed foramen can benefit from mild®.
Are you ready to put your lumbar spinal stenosis patients on the path to long-term relief? Contact Vertos Medical and discover why leading interventionalists offer mild® in their practice.
Denis G. Patterson, MD (00:00)
Hey, this is Dr. Denis Patterson. I’m a physical medicine rehabilitation physician who specializes in interventional pain management. I am located in the greater Reno, Tahoe area. I’m the owner of Nevada Advanced Pain Specialists. I’ve been seeing and treating patients in the greater Reno, Tahoe area now for about 15 years, and I have to say that one of the best treatment options that I’ve had come around within the past decade has been the mild® Procedure.
Prior to this procedure, as an interventional pain physician, we’re more trying to cover up pain or, alleviate pain, but we never really could treat the underlying pathophysiology. And so it was nice that we finally got a tool in our bag of tricks to help patients that we could actually treat their underlying pathophysiology. So, a patient with wear and tear over the years, not only are they going to get a swollen disc, swollen facet joints, but the ligamentum flavum can also encroach and push into the spinal canal. And that’s what the mild® Procedure does, it allows us to have access to get between the lamina, dig out this ligamentum flavum, create a couple more millimeters of space, and in essence, probably decrease the pressure at that level in their spine and help alleviate their neurogenic claudication symptoms.
Being an advocate for this procedure, I talked to multiple physicians around the country, and one of the interesting comments that I’ve heard over the years is that, “Hey, I can give this or I can treat patients with this, but they can only have central canal stenosis.” And so to me, these physicians are looking for what I would call is a unicorn. A unicorn is a patient who has central canal stenosis only due to the ligament being hypertrophied and that’s simply not the case. When you really look back at the literature and the 2-year study that they did, the MiDAS study, what we see is only 5% of the patients in that study only had central canal stenosis. 95% of those patients had multifactorial stenosis and so that means they can have neuroforaminal stenosis, lateral recess stenosis, and central canal stenosis. And what we saw in the MiDAS study is all those patients benefited.
(02:17) But even though we have that data, over the years of training physicians in the mild® Procedure, I continue to hear, “Well they have neuroforaminal stenosis, they don’t have just central canal stenosis so, I think I’m going to pick a competitive product to treat these patients instead of the mild® Procedure.” So, from the MiDAS data that I had talked about earlier, we’d seen that patients with multifactorial stenosis benefited from the mild® Procedure. Myself, Dr. Pryzbylkowski, and Dr. Khemlani wanted to retrospectively look at patients who we had treated with the mild® Procedure and see if we could validate the results of [what] the MiDAS study had shown. And so we retrospectively reviewed 91 patients from our 3 clinics, and this went back to January of 2020. And what we found is that 32 of the patients that we had treated out of the 91 had no neuroforaminal narrowing. Meaning, they had central canal stenosis, which included ligamentum flavum hypertrophy, but they also could have had multifactorial pathology, including disc bulges and facet hypertrophy. And then the other 59 patients out of the 91, besides having multifactorial pathology, also had neuroforaminal narrowing. And then what we ended up doing is retrospectively looking at our results of these patients, who benefited from the mild® Procedure in these groups; did both groups, 1 group, or neither group benefit? And we looked at our results at one and three months after having the procedure done. And if you look under our results, we look at [how] both groups got significant pain reduction and response rates, what we see is that the foraminal narrowing group did better.
86% of these patients had a positive response rate, while only 75% of the patients that had no neuroforaminal narrowing had a pain reduction. So, when you first look at this, you think, “Oh God, this has got to be statistically significant that the foraminal narrowing group benefited more than the no foraminal narrowing group from the mild® Procedure.” But, when you really look at the “P” value, there is no statistical significance between the 2 groups. And so the conclusion that we came to, is that patients with lumbar stenosis, with neurogenic claudication, whether they have central canal stenosis only, or they have multifactorial stenosis only, both groups can benefit from having the mild® Procedure done. And so, all physicians should know that they should not exclude patients with multifactorial stenosis from having this procedure be considered.
Benyamin RM, Staats PS, MiDAS ENCORE Investigators. mild® is an effective treatment for lumbar spinal stenosis with neurogenic claudication: MiDAS ENCORE Randomized Controlled Trial. Pain Physician. 2016;19(4):229-242.
Mekhail N, Costandi S, Abraham B, Samuel SW. Functional and patient-reported outcomes in symptomatic lumbar spinal stenosis following percutaneous decompression. Pain Pract. 2012;12(6):417-425. doi:10.1111/j.1533-2500.2012.00565.x.
2012 data from Health Market Sciences report for Vertos Medical 2013.
Data on file with Vertos Medical.
Staats PS, Chafin TB, Golvac S, et al. Long-term safety and efficacy of minimally invasive lumbar decompression procedure for the treatment of lumbar spinal stenosis with neurogenic claudication: 2-year results of MiDAS ENCORE. Reg Anesth Pain Med. 2018;43:789-794. doi:10.1097/AAP.0000000000000868.
Based on mild® Procedure data collected in all clinical studies. Major complications are defined as dural tear and blood loss requiring transfusion.
MiDAS ENCORE responder data. On file with Vertos Medical.
Jain S, Deer TR, Sayed D, et al. Minimally invasive lumbar decompression: a review of indications, techniques, efficacy and safety. Pain Manag. 2020;10(5). https://doi.org/10.2217/pmt-2020-0037. Accessed June 1, 2020.
Deer TR, Grider JS, Pope JE, et al. The MIST Guidelines: the Lumbar Spinal Stenosis Consensus Group guidelines for minimally invasive spine treatment. Pain Pract. 2019;19(3)250-274. doi:10.1111/papr.12744.
Hansson T, Suzuki N, Hebelka H, Gaulitz A. The narrowing of the lumbar spinal canal during loaded MRI: the effects of the disc and ligamentum flavum. Eur Spine J. 2009;18(5):679-686. doi:10.1007/s00586-009-0919-7.
Treatment options shown are commonly offered once conservative therapies (e.g., physical therapy, pain medications, chiropractic) are not providing adequate relief. This is not intended to be a complete list of all treatments available. Doctors typically recommend treatments based on their safety profile, typically prioritizing low risk/less aggressive procedures before higher risk/more aggressive procedures, but will determine which treatments are appropriate for their patients.
The mild® Procedure is a minimally invasive treatment for lumbar spinal stenosis. As with most surgical procedures, serious adverse events, some of which can be fatal, can occur, including heart attack, cardiac arrest (heart stops beating), stroke, and embolism (blood or fat that migrates to the lungs or heart). Other risks include infection and bleeding, spinal cord and nerve injury that can, in rare instances, cause paralysis. This procedure is not for everyone. Physicians should discuss potential risks with patients. For complete information regarding indications for use, warnings, precautions, and methods of use, please reference the devices’ Instructions for Use.
Patient stories on this website reflect the results experienced by individuals who have undergone the mild® Procedure. Patients are not compensated for their testimonial. The mild® Procedure is intended to treat lumbar spinal stenosis (LSS) caused by ligamentum flavum hypertrophy. Although patients may experience relief from the procedure, individual results may vary. Individuals may have symptoms persist or evolve or other conditions that require ongoing medication or additional treatments. Please consult with your doctor to determine if this procedure is right for you.
Reimbursement, especially coding, is dynamic and changes every year. Laws and regulations involving reimbursement are also complex and change frequently. Providers are responsible for determining medical necessity and reporting the codes that accurately describe the work that is done and the products and procedures that are furnished to patients. For this reason, Vertos Medical strongly recommends that you consult with your payers, your specialty society, or the AMA CPT regarding coding, coverage and payment.
Vertos Medical cannot guarantee coding, coverage, or payment for products or procedures. View our Billing Guide.
Vertos is an equal employment opportunity workplace committed to pursuing and hiring a diverse workforce. We strive to grow our team with highly skilled people who share our culture and values. All qualified applicants will receive consideration for employment without regard to sex, age, color, race, religion, marital status, national origin, ancestry, sexual orientation, gender identity, physical & mental disability, medical condition, genetic information, veteran status, or any other basis protected by federal, state or local law.
Hall S, Bartleson JD, Onofrio BM, Baker HL Jr, Okazaki H, O’Duffy JD. Lumbar spinal stenosis. Clinical features, diagnostic procedures, and results of surgical treatment in 68 patients. Ann Intern Med. 1985;103(2):271-275. doi:10.7326/0003-4819-103-2-271.
Kalichman L, Cole R, Kim DH, et al. Spinal stenosis prevalence & association with symptoms: The Framingham Study. Spine J. 2009;9(7):545-550. doi:10.1016/j.spinee.2009.03.005.
Fukusaki M, Kobayashi I, Hara T, Sumikawa K. Symptoms of spinal stenosis do not improve after epidural steroid injection. Clin J Pain. 1998;14(2):148-151. doi:10.1097/00002508-199806000-00010.
Mekhail N, Costandi S, Nageeb G, Ekladios C, Saied O. The durability of minimally invasive lumbar decompression procedure in patients with symptomatic lumbar spinal stenosis: Long-term follow-up [published online ahead of print, 2021 May 4]. Pain Pract. 2021;10.1111/papr.13020. doi:10.1111/papr.13020
Friedly JL, Comstock BA, Turner JA, et al. Long-Term Effects of Repeated Injections of Local Anesthetic With or Without Corticosteroid for Lumbar Spinal Stenosis: A Randomized Trial. Arch Phys Med Rehabil. 2017;98(8):1499-1507.e2. doi:10.1016/j.apmr.2017.02.029
Pope J, Deer TR, Falowski SM. A retrospective, single-center, quantitative analysis of adverse events in patients undergoing spinal stenosis with neurogenic claudication using a novel percutaneous direct lumbar decompression strategy. J Pain Res. 2021;14:1909-1913. doi: 10.2147/JPR.S304997
Pryzbylkowski P, Bux A, Chandwani K, et al. Minimally invasive direct decompression for lumbar spinal stenosis: impact of multiple prior epidural steroid injections [published online ahead of print, 2021 Aug 4]. Pain Manag. 2021;10.2217/pmt-2021-0056. doi:10.2217/pmt-2021-0056
Abstract presented at: American Society of Pain and Neuroscience Annual Conference; July 22-25, 2021; Miami Beach, FL.
Mobility Matters: Low Back Pain in America, Harris Poll Survey, 2022. View data and full summary here.
Deer TR, Grider JS, Pope JE, et al. Best Practices for Minimally Invasive Lumbar Spinal Stenosis Treatment 2.0 (MIST): Consensus Guidance from the American Society of Pain and Neuroscience (ASPN). J Pain Res. 2022;15:1325-1354. Published 2022 May 5. doi:10.2147/JPR.S355285.

